Example #1: the excited electronic states of chlorine dioxide, OClO
K.A. Peterson and H.-J. Werner, J. Chem. Phys. 105, 9823 (1996)
below: 2-dimensional cut for the 2A1 and 2B2 states restricting to C2v symmetry (crossing allowed)
Note that the 2A1 and 2B2 states are allowed to (and indeed do) cross for these symmetric geometries and that the crossing is
really along a seam (line).
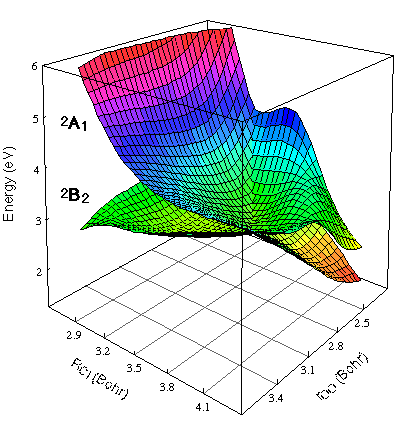
below: 2-dimensional cut for asymmetric bond length displacements
Note that the crossing is now avoided but the surfaces "touch" just at the C2v (r1 = r2) geometries.

Example #2: the excited electronic states of the BrO radical
Note the crossing between the two states of the same symmetry, A2P (Pi) and 32P (Pi), in the hashed region.
In this case the crossing is very narrowly avoided (it's actually depicted below like an allowed crossing) since
the two wavefunctions have very different electronic character. The other curves describe other electronic states
that are allowed to cross the "A" state (dashed lines are quartet states, solid lines are doublet states).

One can explicitly calculate the 1st-order non-adiabatic coupling matrix elements (NACME) as a function of the
internuclear distance R.
Note that the NACME is a very strong function of the internuclear distance and peaks at the crossing point
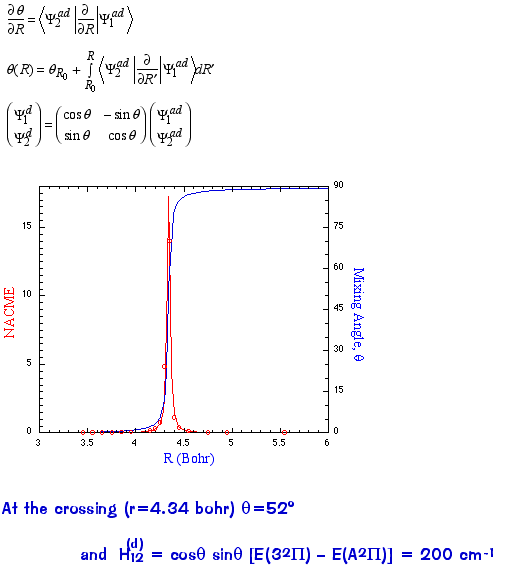
.
The NACME can be used to define what are called "diabatic" states (i.e., non-adiabatic) that differ from the usual "adiabatic"
states (i.e., eigenfunctions of the electronic H) in that their character varies smoothly through the crossing. For two states this
involves just a unitary transformation to the diabatic basis, i.e., just a rotation by the angle q (theta). In this basis the coupling
between the electronic states no longer appears in the nuclear kinetic energy term but only in the coordinates (potential energy
couplings only). The diabatic states are allowed to cross (but have explicit coupling matrix elements, e.g., the H12 term above):