(1) O3 ![]() O2 + O
O2 + O
(2) O3 + O --> 2O2
(1) O3 ![]() O2 + O
O2 + O
(2) O3 + O --> 2O2
a) Adding together the two steps yields: 2O3 --> 3O2
b) The intermediate is O atom since it doesn't appear in the overall reaction
c) For each step:
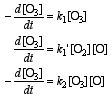
d) The RDS is step 2 since:
a) step 1 is a simple unimolecular reaction
b) the reverse of step 1 does not involve ozone
e) From the result of part (d) and given that the RDS should resemble the empirical rate law,
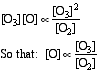
f) Employing the steady state approximation for [O] :
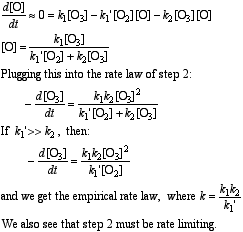
g) To eliminate [O] from the rate law of the RDS (step 2), use the pre-equilibrium of step 1:
